This is an Earthcache
and as such logging it requires special tasks be undertaken.
Failure to complete the assigned tasks will result in the deletion
of your log.
Lime Kilns: High Cliff State
Park
Park information and human history
Between 1,000 and 1,500 years ago, the nomadic Siouan Indians
built effigy mounds here. You can view the four panther-shaped
mounds, two buffalo-shaped mounds, conical mounds, and a linear
mound just a short walk or drive up the hill. In the 1800s this
area was noted for its amazing views of Lake Winnebago and became a
frequently visited site. A limestone quarry operated here for over
100 years until 1956 when the state of Wisconsin bought the land.
In 1957 High Cliff was opened as a state park.
Vehicle admission stickers are required to enter
the park; stickers are available at the park office or use
self-registration pay station when the office is closed. The park
is open for day use from 6 a.m. to 11 p.m. A park map can be found
HERE:
Brief History of the Kilns:
While mining took place here as early as the 1850s, the Western
Lime and Cement Company operated a limestone quarry here from 1895
to 1956. The Kilns you see before you were built to extract the
lime from the stone. At its peak, as many as 40 people worked at
the operation from processing the stone to making barrels to
transport it. Many of these workers were recent immigrants from
Hungary who lived in one of the 16 worker homes in what had become
a small "company town" complete with a telegraph, store, church and
tavern. The store still stands and now serves as a museum and
interpretive center.

The firing process
Simplified, a lime kiln is an oven used to produce quicklime by
the "calcination" of limestone. Limestone is made up mainly of
three components: calcium, carbon and oxygen. When limestone is
heated the carbon escapes as carbon dioxide, leaving lime. This
reaction takes place at 750°F, but a temperature of 1300°F to
2,000°F is usually used to make the reaction proceed more quickly.
The process of lime burning was carried out by a kilnsman who was
experienced in the reduction of limestone. An experienced kilnsman
was required to monitor many variables in order to reduce the
amount of "dead burnt" lime which was not useful as an end product.
The heating and cooling process took several days.
These kilns were "draw" kilns. Draw kilns operate
under the principal of gravity. Limestone is fed into the top of
the kiln and the cooked stone is removed from the bed of the kiln.
Fireplaces were located at the sides of the kiln where fuel was
burnt to cook the limestone. One advantage of the draw kiln was
that it could be operated on a continual basis. Even though this
type of lime kiln was more effective than past versions, it was
still extremely inefficient and the lumber needed to fire it could
easily necessitate the clearing of large tracts of woodland. Fuel
for these three kilns changed from lumber to coal as hardwoods
became increasingly scarce in the area.
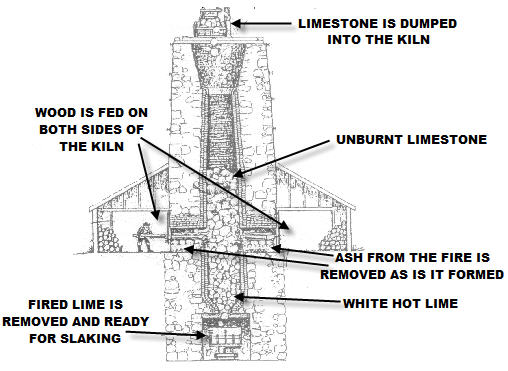
After the lime was fired it was cooled in cooling sheds and
slaked. Slaking involves adding moisture to the lime; this occurred
in a number of possible ways from sprinkling water on the lime to
letting it sit and absorb water from the atmosphere. The correct
mix is approximately one part lime to one part water. Other
additives were combined with the lime to create various
products.
The end days
In the early settlement days the lack of a good system of roads
necessitated the production of lime wherever the raw materials were
easily mined. As transportation methods improved during the 1900s
commercial lime production began to spread throughout the state
wherever limestone was plentiful. Competition, hardwood shortages,
increased transportation costs, and the growing use of Portland
cement, made small-scale kilns became increasingly unprofitable and
most gradually ceased production by the early to mid 1900s. The
final nail in the coffin for many kilns was the beginning of the
Great Depression which saw a near standstill in the construction
industry.
The Niagara Escarpment Rocks!
The limestone (calcium carbonate) cliffs you see here are a part
of the Niagara Escarpment* which continues northeasterly to the
Door County peninsula and on to Niagara Falls. These cliffs were
formed by the settling and hardening of a limy ooze at the bottom
of the shallow Silurian Sea which covered much of Wisconsin around
400 million years ago. This limy ooze was made up of shell and
shell fragments consisting of corals, brachiopods, crinoids and
other types of early life. Many marine organisms extract calcium
carbonate from the seawater to make shells or bones and when these
organisms die their shells and bones accumulate on the seafloor.
Over millions of years these sediments harden into what we see
today as limestone. Calcium carbonate is found naturally as a
component of aragonite, calcite, chalk, marble and travertine.
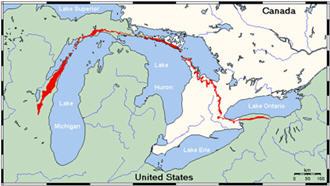
*The Niagara Escarpment shown above in red is the edge of a thick
series of dolomite (sedimentary stone) layers formed during the
Silurian age. The rock is very resistant to erosion and stands up
in relief as a prominent line of bluffs. This steep bluff owes its
prominence to both the resistance of the Silurian dolomite layers
and the relative softness of the Ordovician and Devonian era rocks
on either side.
Uses of Lime / Calcium Carbonate
· As a filler in plastics.
· As an extender in paints.
· As an ingredient of cement.
· To treat animal hides and leather.
· In swimming pools as a pH corrector.
· In agriculture to improve acidic soils.
· In forensic science to reveal fingerprints.
· As a major component of blackboard chalk.
· In adhesives, sealants, and decorating fillers.
· In water and sewage treatment to reduce acidity.
· As a building material in the form of marble or limestone
aggregate.
· Medically as a calcium supplement, an antacid or as a base
material for pill tablets.
· When cured, to create firebrick which was used to line furnaces
and build fire-resistant structures.

|
*** KIDS CORNER ***
A long time ago the cliffs you see here at High Cliff State Park
were under a shallow sea. In this sea lived trillions of tiny
snails and other shelled critters. When these critters died their
shells and bones piled up on the bottom of the sea. Pressure from
other shells, sand and water squashed them together to form a
sedimentary rock known as limestone.
DID YOU KNOW?
Shells are made of calcium just like your teeth!
When limestone is squished really hard it can become
marble. |
LOGGING
REQUIREMENTS:
(Your log may be deleted if you do not follow these logging
requirements)
E-MAIL ME (Do NOT post in your log)
#1 ESTIMATE: How high are each of the three
kilns?
#2 Where was the poor quality rock sent to for processing?
#3 Name the place where the Quick-lime was barreled and
bagged.
POST WITH YOUR FOUND IT LOG
#4 Upload a photo taken at a recognizable
part of the site and include your GPS in the picture.
The Geocache Notification Form has
been submitted to Jason Wiese, Park Manager. Geocaches placed on
Wisconsin Department of Natural Resouce managed lands require
permission by means of a notification form. Please print out a
paper copy of the notification form, fill in all required
information, then submit it to the land manager. The DNR
Notification form and land manager information can be obtained at:
http://www.wi-geocaching.com/modules.php?name=Wiki&pagename=Hiding%20A%20Cache
References:
http://en.wikipedia.org/wiki/Lime_kiln
http://en.wikipedia.org/wiki/Niagara_Escarpment
http://www.limehousekilns.ca/history.htm
http://www.co.ozaukee.wi.us/history/LimeKilns.htm
http://www.mpm.edu/collections/learn/reef/grafton-front.html
http://encarta.msn.com/encnet/refpages/RefArticle.aspx?refid=761565838
http://dnr.wi.gov/org/land/parks/specific/highcliff/history.html
http://www.friendsofhighcliff.org/history.html