Hooper Springs

The city of Soda Springs is named for the thousands of natural
springs of carbonated water that are located in and around the
city. The most notable is Hooper Spring.
Hooper Spring is a natural spring that rises to the surface of
the Earth in a little pool here. The spring water then travels into
a brook leaving the spring house. This particular spring is
carbonated as it reaches the surface.
Carbonation
Carbonation occurs when carbon dioxide is dissolved in water or
an aqueous solution. This process yields the "fizz" to carbonated
water and sparkling mineral water, the head to beer, and the cork
pop and bubbles to champagne and sparkling wine. It is also behind
the Diet Coke and Mentos eruption effect.
Effervescence
Effervescence is the escape of gas from an aqueous solution. The
term is used to describe the foaming or fizzing that results from a
release of gas. In the lab, a common example of effervescence is
the addition of hydrochloric acid to a block of limestone. If a few
pieces of marble or an antacid tablet are put in hydrochloric acid
in a test tube fitted with a cork, effervescence of carbon dioxide
can be witnessed. This process is generally represented by the
following reaction, where a pressurized dilute solution of carbonic
acid in water releases gaseous carbon dioxide at decompression:
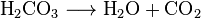
In simple terms, it is the result of the chemical reaction
occurring in the liquid which produces a gaseous product.
Fizz
"Fizz" is a word that is used to describe the action or sound of
gas bubbles moving through and escaping from a liquid. Fizz also
describes the formation of a foam of this gas and liquid at the top
of the liquid's container. The word itself is an example of
onomatopoeia, derived from the sound the multiple bubbles make
together as they "pop" when they escape. A carbonated beverage,
such as cola or beer, will form bubbles when the dissolved carbon
dioxide is depressurized to form emulsions at the top, and it will
make "fizzing" sounds when it is opened or poured into a container.
In the United Kingdom, soft drinks are often referred to as 'fizzy
drinks'. A cocktail based on carbonated water and an acidic juice
is called a Fizz, such as the Gin Fizz. Fizz can also result from a
chemical reaction, such as a solid dissolving in a liquid to
produce gas. For example, Alka-Seltzer brand tablets, used to treat
stomach indigestion, form an effervescent solution that fizzes when
dropped into water. The essential chemical reaction is:
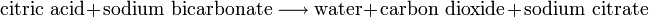

Shaking
It is commonly said that shaking a carbonated beverage will
cause large amounts of foam to erupt upon opening, and it is often
believed that shaking a bottle containing a carbonated beverage
will cause the pressure inside to rise. In fact, when a pressure
gauge is attached to a pressurized bottle of a carbonated beverage,
it is found that the pressure within does not increase. It is
instead the formation of tiny bubbles from the agitation that
causes the foam; upon opening, the size of the bubbles will rapidly
increase due to the reduction in pressure, resulting in excessive
foaming. Agitating carbonated liquid in a resealed vessel increases
the rate that CO2 is released from the solution and the rate that
it approaches equilibrium pressure. The equilibrium pressure
depends on the temperature, composition, and purity of the liquid
and is not affected by agitation.
Measuring Carbonation
The quality of carbonated beverages including softdrinks,
seltzer and beer is affected by the amount of dissolved CO2 (the
gas that causes carbonation) and the amount of carbonic acid in the
drink. Carbon dioxide (CO2) has an infrared absorption wavelength
of 4.27um and can be measured online using an infrared carbonation
sensor. This is an improvement to the traditional inferred
measurement method using temperature and pressure for Henry's Law
coefficients because this methodology is influenced by changes in
density and alcohol content. Infrared measurements are not affected
by changes in density or alcohol content because they are actually
measuring the CO2 molecule using Beer Lamberts Law. The amount of
carbonation in a beverage is measured in Volumes or grams/liter.
This is because introducing CO2 into a beverage will change its
weight. An easy experiment to prove this is to take a seltzer
bottle and weigh it. Carefully remove the top slowly so no liquid
escapes from the bottle as the gas escapes the weight of the bottle
of seltzer will go down. Shaking the bottle closed and then opening
it to remove more CO2 will increase this effect.
Natural and Artificial
Carbonation
Carbonation can occur as a result of natural processes: when
yeast ferments dissolved sugars sealed in a pressure-tolerant
bottle or keg; when underground volcanic carbon dioxide carbonates
well water; or when rainwater passes through limestone into a cave
and forms a stalactite. Or it can be done artificially by
dissolving carbon dioxide under pressure into the liquid. Sometimes
natural carbonation is called conditioning while the term
carbonation is reserved for the artificial process.
Uses
In many consumer beverages such as soft drinks (well known
examples include Coca-Cola, 7 Up and Pepsi), carbonation is used to
give "bite". Contrary to popular belief, the fizzy taste is caused
by dilute carbonic acid inducing a slight burning sensation, and is
not caused by the presence of bubbles. This can be shown by
drinking a fizzy drink in a hyperbaric chamber at the same pressure
as the beverage. This gives much the same taste, but the bubbles
are completely absent. Carbonation is sometimes used for reasons
other than consumption for example: to lower the pH (raise the
hydrogen ion concentration) of a water solution, and in the
cleaning industry (Chem-Dry and Carbonated Solutions both use
carbonated cleaning solutions for carpet cleaning).
Brewing
In homebrewing, overcarbonation can be dangerous; it can result
in bottles gushing or even exploding. Adding priming sugar or malt
extract at bottling time to beer that has had its fermentable sugar
content totally consumed is the safest approach to carbonation.
Exceeding recommended levels of priming sugar for a given recipe is
dangerous, as is using inappropriate bottles or improper capping
methods. Beer may also be force-carbonated using a keg and special
bottling equipment so that the carbonation level can be carefully
controlled.

This natural occurance of carbonation is one of nature's
phenomenums. It makes for a great natural "soda" when mixed in a
jug with some sugar and your favorite Kool-aid mix. I spent many
years as a kid coming to Soda to visit and grabbing some of the
home-made soda that mom would make.
Free, clear sprakling soda water is still available in a
beautiful Soda Springs city park located two miles north from the
center of town. A prime attraction for more than 160 years, soda
water from these springs was marked nationally after rail service
reached this resort area in 1882. W.H. Hooper, Salt Lake City's
leading banker and president of ZCMI (Zions Cooperative Mercantile
Institution), had his summer home here. He did much to found and
promote Soda Springs and its soda water industry while serving as
Utah's delegate to Congress.
To log this EarthCache, please take a
photo of your GPS, or someone in your group, at the Hooper Springs
Spring House and post to the log. Also answer this question in an
email to me:
What color is the ground under the water of
the brook as it leaves the spring house?
Do not post the answer of this question,
even encrypted, in your log or it will have to be
deleted.
While in Soda Springs make sure you stop by and see the:
Soda Springs Geyser

Not a nature-made phenomenum but VERY COOL just the same!
Soda Springs has the only captive geyser in the world. It was
discovered in an attempt to find a hot watter source for a swimming
pool. On November 30, 1937, the drill went down 315 feet and
unleashed the geyser, which is now a man-made carbon dioxide
generated cold water geyser. The extreme pressure is caused by
carbon dioxide gas mixing with water in an underground chamber. It
is now capped and controlled by a timer. It erupts every hour on
the hour. The geyser reaches heights of 100 feet year
round.