Cache is located at the Green Lake PWA
May get wet feet during a rainy spring or summer.
Use stealth during the boating season.
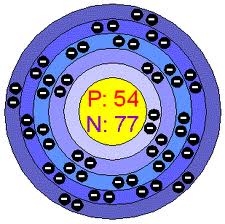
History: Xenon was discovered by Sir William
Ramsay and Morris Travers in 1898 in the residue left after
evaporating liquid air components. Krypton and neon had been
discovered by the same methods by the same workers only weeks
earlier. They had to work with huge volumes of air to produce just
a little xenon since it turned out that xenon is only present to
the extent of about 0.087 ppm in the atmosphere.
Before 1962, it was generally assumed that xenon and other noble
gases were unable to form compounds. Among the compounds of xenon
now reported are xenon hydrate, sodium perxenate, xenon deuterate,
difluoride, tetrafluoride, hexafluoride, and XePtF6 and
XeRhF6. The highly explosive xenon trioxide,
XeO3, is known.
Metallic xenon is produced by applying several hundred kilobars
of pressure. Xenon in a vacuum tube produces a blue glow when
excited by an electrical discharge and finds use in strobe lamps.
It is an odourless, colourless, inert gas.
Sources: Xenon is present to a small extent in the
atmosphere (less than 1 ppm by volume) and is obtained as a
byproduct from the liquefaction and separation of air. This would
not normally be carried out in the laboratory and xenon is
available commercially in cylinders at high pressure.
Uses:
- Used in making electron tubes, stroboscopic lamps, bactericidal
lamps, and lamps used to excite ruby lasers for generating coherent
light
- Used in the atomic energy field in bubble chambers, probes, and
other applications where its high molecular weight is of value
- Uotentially useful as a gas for ion engines
- The perxenates are used in analytical chemistry as oxidizing
agents