The coordinates will take you to a specimen full of Fe and some other things too. This particular specimen is from Marquette, Michigan. The upper peninsula is well known for its high quality iron deposits, as well as other metals such as copper.
To log this Earthcache, please email the CO with answers to the following questions:
1. Why do you think there are different layers (lines) of material and not just one band?
2. Describe the different layers and provide your thinking as to why the layers look like they do (color, direction, width, straight/wavy)
3. If you brought a magnet with you, which layers did the magnet stick to?
4. Any surprises?
Please do not post answers to the questions within your log entry, nor pictures of the specimen this earthcache focuses on..
Types of rocks
Rocks are categorized in three major groupings: Igneous (formed when magma cools), Metamorphic (formed by heat and pressure), and sedimentary (formed over time as layers of sediment harden into rock).
Iron ore is a chemical sedimentary rock. Elemental iron in solution (dissolved in water) combines with oxygen, solidifies, and settles as sediment. Common iron ores are Hematite and Magnetite. Sometimes these ores are represented by larger veins of solid ore, other times they appear as banded iron formations (BIFS). BIFs are a distinctive sedimentary rock consisting of alternating layers of iron oxides (such as magnetite or hematite) and other materials (e.g., chert, quartz, shale).
Origin of BIFs
Most BIFs formed very early in the history of the earth (Precambrian), when the oceans were more acidic and there was little to no atmospheric oxygen. These two conditions were crucial for the creation of BIFs. Neutral or acidic waters allowed for iron to be soluble (dissolved) more easily. The lack of oxygen allowed for the iron to remain in the ocean until a new supply of oxygen was available to join with the elemental iron. Many scientists believe this oxygen source was blue green algae photosynthesis (cyanobacteria), although there is some disagreement in the scientific community that microbial activity played a direct role in the formation of BIFs. Other free oxygen theories include oxidation by anerobic bacteria and the freeing of oxygen by UV radiation.
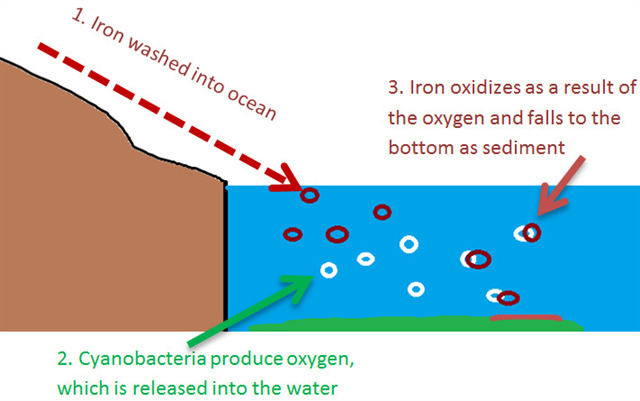 The prevalent theory suggests algae released oxygen that reacted with the dissolved iron, causing it to precipitate (become solid) and sink to the bottom of the ocean floor.
The prevalent theory suggests algae released oxygen that reacted with the dissolved iron, causing it to precipitate (become solid) and sink to the bottom of the ocean floor.
References:
https://www.princeton.edu/~achaney/tmve/wiki100k/docs/Banded_iron_formation.html
http://geology.com/rocks/sedimentary-rocks.shtml
http://ammin.geoscienceworld.org/content/90/10/1473.short
http://www.youtube.com/watch?v=ylB3r7cOtY4
Smithsonian BIF Earthcache: http://coord.info/GC3RR2C
Schaetzl, R., Darden, J., & Brandt, D. (2009). Michigan Geography and Geology. New York: Pearson.
Congrats GeoEagleScout, FTF