The cache is not at the posted coordinates. You will have to solve the puzzle to find the cache.

The Periodic Table is one of, if not the, most important tools in all of chemistry. Elements are grouped together by properties in blocks and columns. Trends such as atomic size or electronegativity can increase or decrease as you move up or down and left or right in the table. You may want to get crazy and research diagonal trends! But, we are not here to teach you chemistry, that is why college, wikipedia, and that guy on the Nat. Geo channel exist. Today, we are going to look at some basic things in the periodic table that could be used for geocaching puzzles (but not geo flash mobs, new virtuals, or new webcams since those fun things are illegal no-no's).
Puzzle Considerations
What information could we use to make a puzzle from the Periodic Table? The easiest method is to use the elemental symbols to spell words that are related to the coordinates or just use a simple substitution of elemental symbol and atomic number. The posted puzzle coordinates might look like:
N Mo Ga.HoB W Bi O.NSb is converted to N 42° 31.675 W 083° 08.751
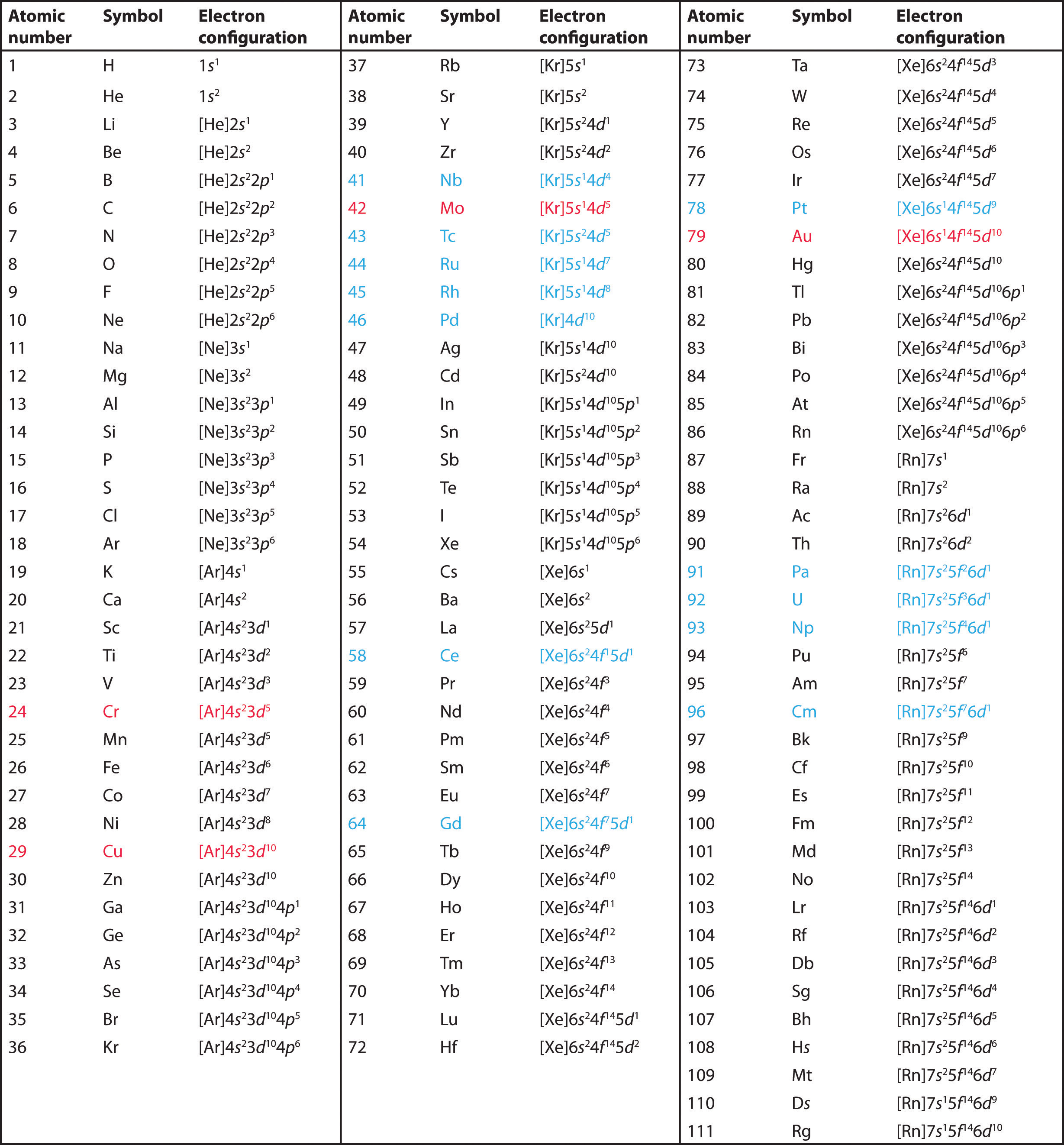
Do you see where the numbers came from? I used the atomic number from the elements. The atomic number is the number at the top of each element's "rectangle." The average atomic mass is the number at the bottom of the "rectangle."
Here are some other items to consider when working with Periodic Table puzzles.
- The atomic number is equal to the number of protons in the element. An element can have different numbers of neutrons in its nucleus which leads to the topic of isotopes. Isotopes are present in different ratios or abundances in nature. What if I was looking for a particular abundance for a specific isotope in a puzzle?
-
For Example: Let's look at a little puzzle with Ruthenium and Tin isotopes
(102Ru).(96Ru+98Ru)(96Ru+98Ru+98Ru)(96Ru) & (119Sn).(117Sn)(124Sn)(122Sn-3) can be translated to 31.675 & 8.751
Here is a link for you to examine the isotope abundance values for Tin (see if you can figure out the origin of the Ruthenium values)
- Many of the columns in the Periodic Table have specific names. For example, the first column elements are known as the Alkali Metals and the far right column are known as the Noble Gases. I could use these designations to count letters and get some numbers for a puzzle (also you will most likely see addition or subtraction of integers or other elements in these types of problems)
-
For Example: Let's look at this puzzle: (Cl-Ar)(Na-Ne).Li(Mg-Na)Ne F.(Ca-K)He(Li-Ne)
Again, the answer is 31.675 & 8.751. Here we used the location of the element and its Group (column) name to solve the puzzle (Alkali = 6, Noble = 5, Alkaline Earth = 13, Chalcogens = 10, and Halogens = 8).
Here is a link to look at Groups of the Periodic Table (you are on your own for the math)
- Each element has a quantity of electrons equal to the atomic number. These electrons fill orbitals around the nucleus (protons + neutrons) in a very specific way. This specific filling is called the electron configuration of the element. There are many numbers involved in these configurations which could be ripe for a puzzle.
-
For Example: Let's try to solve this puzzle: NaH.CsFrRb (K+K).FrRbH
Again, the answer is 31.675 & 8.751. Here we used the highest energy level of the outermost electron to solve the puzzle.
That is a good place to start with your Periodic puzzles. One day, perhaps we'll see Periodic Puzzle Solving 201!
Now. . .The Cache Puzzle
VScScCoTb NiScNiFe
