PEI Zinc - Woodward cemetery EarthCache
PEI Zinc - Woodward cemetery
-
Difficulty:
-

-
Terrain:
-

Size:  (other)
(other)
Please note Use of geocaching.com services is subject to the terms and conditions
in our disclaimer.
PEI Zinc - Woodward cemetery
The Geology of Zinc:
Zinc is never found as the free metal. It makes up about .0075% of the Earth's crust, making it the 24th most abundant element. Its resources total about 1.9 billion tonnes worldwide. It is the first element in Group 12 element Periodic table. In some respects zinc is chemically similar to Magnesium.
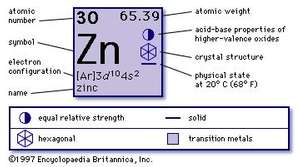
Physical Properties:
Zinc is somewhat less dense than iron and has a hexagonal crystal structure, with a distorted form of hexagonal close packing. The metal is hard and brittle at most temperatures but becomes malleable between 100 and 150 °C. Above 210 °C, the metal becomes brittle again and can be pulverized by beating. Zinc is a fair conductor of electricity.
Many alloys contain zinc, including brass. Other metals long known to form binary alloys with zinc are aluminium, antimony, bismuth, gold, iron, lead, mercury, silver, tin, magnesium, cobalt, nickel, tellurium, and sodium. A bar of zinc generates a characteristic sound when bent, similar to tin cry.
Using Zinc as Tombstones:
White Bronze (Zinc) monuments were made for only 40 years. Unlike traditional gravestones and markers, these were only sold through company catalogues and in person by a sales agent. Sales hit their peak in the 1880s. In an attempt to boost sales, they changed the "finish" on the metal base so it would resemble stone. The sandblasting roughened the surface and speed up the natural formation of protective oxide coating. Many pre 1879 monuments are smooth with a grayish colour while the post 1879 monuments have rough surface and are more bluish-silver in colour. By WWI, production was shut down as all zinc was needed for the war effort.
Monuments
The most common damage to zinc cemetery monuments is breakage of the brittle metal and separation at seams where components were joined at the foundry. This can usually be remedied by soldering and other metallic repair techniques or by using epoxy or polyester resins.
Zinc is subject to "creep" (permanent deformation over time), which may become visible when loadings are appreciable. Irreparable damage invariably results, however, after concrete can be poured inside sagging monuments in an attempt to provide support.

Corrosion is a potential problem for any metal monument, especially in highly polluted or seaside atmospheres. Nevertheless, white-bronze monuments, which were meant to remain unpainted, survive remarkably well. Perhaps this is because the cast metal was relatively pure (more than 99% zinc) and the joining metal was also composed of zinc.
To log this Earthcache please answer the following questions and send answers in a timely manner to my geocaching profile or email. Answers not received will result in deleted logs. Do not post your answers in your log.
Questions:
1. Is the monument solid or hollow? Try tapping lightly.
2. As "creeping" is a big problem with zinc monuments, do you see any evidence on this one? Or evidence of how this monument is holding up?
3. How tall is this monument.?
4. Provide name of two of the other three zinc monuments in cemetery?
5. Post a picture of the monument or one of the other zinc monuments.
Additional Hints
(No hints available.)