Fluorine is the ninth element on the periodic table. It is the 13th most abundant element on earth but due to its highly reactive nature (it is the most electronegative of the elements), it is not found naturally in its standard state (F2). Instead, throughout nature, fluorine is found as an anion (fluoride, F-). Fluorine is essential to human life and about 3 mg of flourine (as fluoride) can be found in the average human body.

Figure 1: The mineral flourite, CaF2. (Image credit: https://www.livescience.com/28779-fluorine.html)
Flourine and it's compounds can be both incredibly dangerous, but extremely useful. For example, hydroflouric acic (HF) is extremely toxic, but is used extensively in industry to etch glass. It's toxicity is so great that even a few drops on your skin can kill you. But when flourine is incorporated into the backbones of drugs and other therapeutics, the flourine derivatized compounds can show increased effectiveness, including higher metabolic stability, increased binding to target molecules, and enhanced membrane permeability. Accoring to a 2020 article in ACS Omega, an estimated 20% of current pharmeceuticals contain at least one flourine atom.
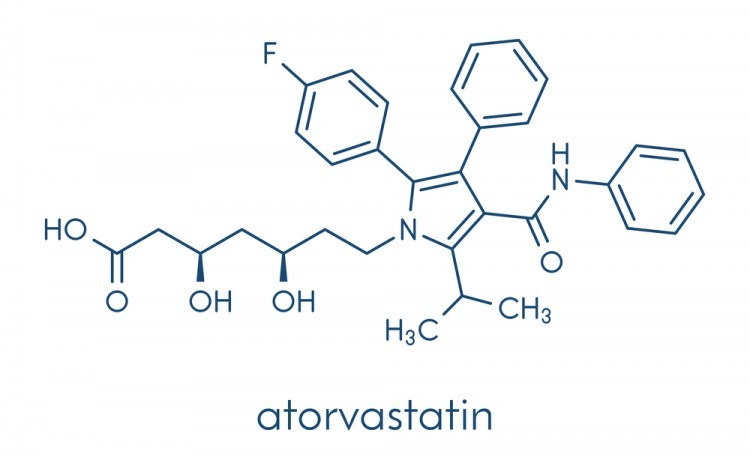
Figure 2 - The structure of Lipitor, a flourine containing medication used to control high cholesterol. (Image credit: https://walrus.com/questions/does-lipitor-atorvastatin-cause-hair-loss)
Cache information: Log only, BYOP. Eppendorf tube with a magnet attached.
Sources:
https://pubs.acs.org/doi/10.1021/acsomega.0c00830
https://emergency.cdc.gov/agent/hydrofluoricacid/basics/facts.asp
https://www.livescience.com/28779-fluorine.html