Salt Of Awaba EarthCache
-
Difficulty:
-

-
Terrain:
-

Size:  (other)
(other)
Please note Use of geocaching.com services is subject to the terms and conditions
in our disclaimer.
Welcome to Lake Macquarie
To complete this earthcache, you will need to answer the questions below, as well as complete a field test. To complete the field test, you will need either a bottle or container calibrated to 1L, or an EC meter or similar to measure the salinity of the water.
Lake Macquarie, or otherwise known as Awaba meaning 'a plain surface', is Australia's largest coastal salt water lagoon. Covering approximately 110 sq kms and a catchment area of over 600 sq kms, Lake Macquarie contains 12 major freshwater tributaries, making it an estuarine lake. It is connected to the sea by Swansea Channel and Lakes Entrance, and although this connection was once a natural intermittent opening, it has been made permanently open by man made intervention.
Lake Macquarie was formed approximately 6,000 years ago, and was once a dry valley until our climate changed around the end of the Ice Age approximately 8,000-10,000 years ago that saw sea levels rise resulting in flooding, and the inundation of our original coast line. Estuarine lakes are defined as a partly enclosed coastal body of brackish water with one or more rivers or streams flowing into it, and with a free connection to the open sea. 'Brackish water' is water that has more salinity than fresh water, but not as much as seawater.
Seawater
Seawater, or salt water, is water from a sea or ocean, but is ultimately formed from the rocks on land. When rain falls, it carries with it some dissolved carbon dioxide from the surrounding air, which causes the rain to be slightly acidic. The rain physically erodes the rocks by the acids chemically breaking them down causing salts and minerals to be carried along in a dissolved state as ions. These ions then runoff into our freshwater tributaries, and then into the ocean. Many of these dissolved ions are used up by various organisms in the ocean, however, others are not, and are left for long periods of time where the concentration of them build up. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, or 599 mM), which is a specific gravity of about 1.025. This means that every kilogram of seawater has approximately 35 grams of dissolved salts (predominantly sodium and chloride). Seawater is denser than both fresh water and pure water because the dissolved salts increase the mass by a larger proportion than the volume.
As Lake Macquarie is connected to the sea, and derives it's salinity from the sea, the term 'thalassic' can be used. The term 'thalassic' is used to refer to any smaller body of water relating to the sea or ocean. For bodies of water that have a relatively stable or constant salinity measure, they are defined as 'homoiohaline, whereas waters that are unstable or variable can be described as being poikilohaline. A modified version of the 'Venice' system, used for classifying bodies of water based on their salinity, is shown below.
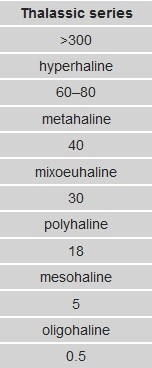
Image courtesy of wikipedia
Salinity measurement
There are two main methods of determining the salt content of water: Total Dissolved Salts (or Solids) and Electrical Conductivity.
Total Dissolved Salts (TDS) is measured by evaporating a known volume of water to dryness, then weighing the solid residue remaining. Electrical conductivity (EC) is measured by passing an electric current between two metal plates (electrodes) in the water sample and measuring how readily current flows (ie conducted) between the plates. The more dissolved salt in the water, the stronger the current flow and the higher the EC. Measurements of EC can be used to give an estimate of TDS.
Measuring Total Dissolved Salts (TDS)
To calculate Total Dissolved Salts, you will need to accurately measure 1L of water. To calibrate your bottle to 1L (1000mls), collect your water sample, and weigh it on a set of scales to equal 1k (1000g). Remember to factor in the weight of your bottle/container when you do this.
1000mls = 1000g.
Pour the water into a pot, and place it on a stove to evaporate ALL the water. Carefully remove the remaining salt left behind, and use the following formula to calculate TDS.
Salinity = salt mass (g) / 1000mL
Multiply your result by 100 to turn it into a percentage.
Logging Requirements:
To log this earthcache, please email me the answers to the following questions!
1.a. What is the salinity of the water at the published coordinates? (Please refer above for methods on how to do this)
b. Is this reading above or below the average salinity for seawater? Explain the reasons that may effect this result.
2. In looking at the two reference points (see the additional waypoints below), would you predict any differences in salinity readings? Provide the rationale for your prediction.
3. Based on your salinity reading at the published coordinates and using the 'Venice' system above, what classification would you give to Lake Macquarie?
Please feel free to log your visit online before receiving confirmation of your answers from me, as I will contact you if there is a problem with any of them. If your email is not received within a timely manner of your online log...then your log will be deleted.
My thanks goes to stegan for the inspiration in developing this earthcache.
References:
http://www.depi.vic.gov.au
http://www.famer.unsw.edu.au
http://www.dpi.nsw.gov.au
http://www.newcastle.edu.au
http://water.usgs.gov
Additional Hints
(No hints available.)